Jump to navigation
Jump to search
NCBI: 10-JUN-2013
⊟Summary[edit | edit source]
- organism: Staphylococcus aureus COL
- locus tag: SACOL0975 [new locus tag: SACOL_RS04990 ]
- pan locus tag?: SAUPAN003107000
- symbol: SACOL0975
- pan gene symbol?: cdr
- synonym:
- product: coenzyme A disulfide reductase
⊟Genome View[edit | edit source]
⊟Gene[edit | edit source]
⊟General[edit | edit source]
- type: CDS
- locus tag: SACOL0975 [new locus tag: SACOL_RS04990 ]
- symbol: SACOL0975
- product: coenzyme A disulfide reductase
- replicon: chromosome
- strand: -
- coordinates: 981069..982385
- length: 1317
- essential: unknown other strains
⊟Accession numbers[edit | edit source]
- Gene ID: 3237687 NCBI
- RefSeq: YP_185843 NCBI
- BioCyc: see SACOL_RS04990
- MicrobesOnline: 912444 MicrobesOnline
⊟Phenotype[edit | edit source]
Share your knowledge and add information here. [edit]
⊟DNA sequence[edit | edit source]
- 1
61
121
181
241
301
361
421
481
541
601
661
721
781
841
901
961
1021
1081
1141
1201
1261ATGCCCAAAATAGTCGTAGTCGGAGCAGTCGCTGGCGGTGCAACATGTGCCAGCCAAATT
CGACGTTTAGATAAAGAAAGTGACATTATTATTTTTGAAAAAGATCGTGATATGAGCTTT
GCTAATTGTGCATTGCCTTATGTCATTGGCGAAGTTGTTGAAGATAGAAGATATGCTTTA
GCGTATACACCTGAAGAATTTTATGATAGAAAGCAAATTACAGTAAAAACTTATCATGAA
GTTATTGCAATCAATGATGAAAGACAAACTGTATCTGTATTAAATAGAAAGACAAACGAA
CAATTTGAAGAATCTTACGATAAACTCATTTTAAGCCCTGGTGCAAGTGCAAATAGCCTT
GGCTTTGAAAGTGATATTACATTTACACTTAGAAATTTAGAAGACACTGATGCTATCGAT
CAATTCATCAAAGCAAATCAAGTTGATAAAGTATTGGTTGTAGGTGCAGGTTATGTTTCA
TTAGAAGTTCTTGAAAATCTTTATGAACGTGGTTTACACCCTACTTTAATTCATCGATCT
GATAAGATAAATAAATTAATGGATGCCGACATGAATCAACCTATACTTGATGAATTAGAT
AAGCGGGAGATTCCATACCGTTTAAATGAGGAAATTAATGCTATCAATGGAAATGAAATT
ACATTTAAATCAGGAAAAGTTGAACATTACGATATGATTATTGAAGGTGTCGGTACTCAC
CCCAATTCAAAATTTATCGAAAGTTCAAATATCAAACTTGATCGAAAAGGTTTCATACCG
GTAAACGATAAATTTGAAACAAATGTTCCAAACATTTATGCAATAGGCGATATTGCAACA
TCACATTATCGACATGTCGATCTACCGGCTAGTGTTCCTTTAGCTTGGGGCGCTCACCGT
GCAGCAAGTATTGTTGCCGAACAAATTGCTGGAAATGACACTATTGAATTCAAAGGCTTC
TTAGGCAACAATATTGTGAAGTTCTTTGATTATACATTTGCGAGTGTCGGCGTTAAACCA
AACGAACTAAAGCAATTTGACTATAAAATGGTAGAAGTCACTCAAGGTGCACACGCGAAT
TATTACCCAGGAAATTCCCCTTTACACTTAAGAGTATATTATGACACTTCAAACCGTCAG
ATTTTAAGAGCAGCTGCAGTAGGAAAAGAAGGTGCAGATAAACGTATTGATGTACTATCG
ATGGCAATGATGAACCAGCTAACTGTAGATGAGTTAACTGAGTTTGAAGTGGCTTATGCA
CCACCATATAGCCACCCTAAAGATTTAATCAATATGATTGGTTACAAAGCTAAATAA60
120
180
240
300
360
420
480
540
600
660
720
780
840
900
960
1020
1080
1140
1200
1260
1317
⊟Protein[edit | edit source]
⊟General[edit | edit source]
- locus tag: SACOL0975 [new locus tag: SACOL_RS04990 ]
- symbol: SACOL0975
- description: coenzyme A disulfide reductase
- length: 438
- theoretical pI: 5.05283
- theoretical MW: 49290.5
- GRAVY: -0.310502
⊟Function[edit | edit source]
- reaction: EC 1.8.1.14? ExPASyCoA-disulfide reductase 2 CoA + NADP+ = CoA-disulfide + NADPH
- TIGRFAM: Cellular processes Detoxification CoA-disulfide reductase (TIGR03385; EC 1.8.1.14; HMM-score: 687)and 20 moreCentral intermediary metabolism Nitrogen metabolism nitrite reductase [NAD(P)H], large subunit (TIGR02374; EC 1.7.1.4; HMM-score: 105.7)dihydrolipoyl dehydrogenase (TIGR01350; EC 1.8.1.4; HMM-score: 104.4)Cellular processes Detoxification mercury(II) reductase (TIGR02053; EC 1.16.1.1; HMM-score: 70.8)Unknown function Enzymes of unknown specificity putative bacillithiol system oxidoreductase, YpdA family (TIGR04018; EC 1.8.-.-; HMM-score: 63.5)Energy metabolism Electron transport thioredoxin-disulfide reductase (TIGR01292; EC 1.8.1.9; HMM-score: 63.4)Energy metabolism Electron transport glutathione-disulfide reductase (TIGR01421; EC 1.8.1.7; HMM-score: 59.5)pyridine nucleotide-disulfide oxidoreductase family protein (TIGR03169; HMM-score: 57.3)trypanothione-disulfide reductase (TIGR01423; EC 1.8.1.12; HMM-score: 54)Energy metabolism Electron transport glutathione-disulfide reductase (TIGR01424; EC 1.8.1.7; HMM-score: 52.6)thioredoxin and glutathione reductase (TIGR01438; EC 1.6.4.-; HMM-score: 48.1)putative alkyl hydroperoxide reductase F subunit (TIGR03143; EC 1.6.4.-; HMM-score: 47.2)mycothione reductase (TIGR03452; EC 1.8.1.15; HMM-score: 42.5)Cellular processes Detoxification alkyl hydroperoxide reductase subunit F (TIGR03140; EC 1.8.1.-; HMM-score: 30.5)Cellular processes Adaptations to atypical conditions alkyl hydroperoxide reductase subunit F (TIGR03140; EC 1.8.1.-; HMM-score: 30.5)Amino acid biosynthesis Glutamate family glutamate synthase (NADPH), homotetrameric (TIGR01316; EC 1.4.1.13; HMM-score: 29.3)putative selenate reductase, YgfK subunit (TIGR03315; HMM-score: 25.6)glutamate synthase, small subunit (TIGR01318; HMM-score: 15.5)glutamate synthase, NADH/NADPH, small subunit (TIGR01317; EC 1.4.1.-; HMM-score: 12.7)Protein synthesis tRNA and rRNA base modification tRNA U-34 5-methylaminomethyl-2-thiouridine biosynthesis protein MnmC, C-terminal domain (TIGR03197; HMM-score: 12.5)Unknown function Enzymes of unknown specificity flavoprotein, HI0933 family (TIGR00275; HMM-score: 11.5)
- TheSEED :
- CoA-disulfide reductase (EC 1.8.1.14)
- PFAM: NADP_Rossmann (CL0063) Pyr_redox_2; Pyridine nucleotide-disulphide oxidoreductase (PF07992; HMM-score: 177)and 12 morePyr_redox; Pyridine nucleotide-disulphide oxidoreductase (PF00070; HMM-score: 63.5)Pyr_redox_3; Pyridine nucleotide-disulphide oxidoreductase (PF13738; HMM-score: 49.2)Reductase_C (CL0608) Pyr_redox_dim; Pyridine nucleotide-disulphide oxidoreductase, dimerisation domain (PF02852; HMM-score: 39.1)NADP_Rossmann (CL0063) DAO; FAD dependent oxidoreductase (PF01266; HMM-score: 18.4)NAD_binding_8; NAD(P)-binding Rossmann-like domain (PF13450; HMM-score: 16.2)NAD_binding_7; Putative NAD(P)-binding (PF13241; HMM-score: 15.9)Lys_Orn_oxgnase; L-lysine 6-monooxygenase/L-ornithine 5-monooxygenase (PF13434; HMM-score: 15.7)RNase_H (CL0219) DDE_Tnp_1; Transposase DDE domain (PF01609; HMM-score: 15.5)NADP_Rossmann (CL0063) Shikimate_DH; Shikimate / quinate 5-dehydrogenase (PF01488; HMM-score: 13.1)MurF-HprK_N (CL0365) MurD-like_N; Mur ligase MurD-like, N-terminal domain (PF21799; HMM-score: 13.1)NADP_Rossmann (CL0063) HI0933_like; HI0933-like protein Rossmann domain (PF03486; HMM-score: 12.9)FAD_binding_2; FAD binding domain (PF00890; HMM-score: 11)
⊟Structure, modifications & cofactors[edit | edit source]
- domains:
- modifications:
- cofactors: FAD
- effectors:
⊟Localization[edit | edit source]
- PSORTb: Cytoplasmic
- Cytoplasmic Score: 9.97
- Cytoplasmic Membrane Score: 0
- Cellwall Score: 0.01
- Extracellular Score: 0.02
- Internal Helices: 0
- DeepLocPro: Cytoplasmic
- Cytoplasmic Score: 0.9705
- Cytoplasmic Membrane Score: 0.0257
- Cell wall & surface Score: 0.0002
- Extracellular Score: 0.0037
- LocateP: Intracellular
- Prediction by SwissProt Classification: Cytoplasmic
- Pathway Prediction: No pathway
- Intracellular possibility: 0.83
- Signal peptide possibility: -0.5
- N-terminally Anchored Score: 1
- Predicted Cleavage Site: No CleavageSite
- SignalP: Signal peptide LIPO(Sec/SPII) length 15 aa
- SP(Sec/SPI): 0.027763
- TAT(Tat/SPI): 0.001484
- LIPO(Sec/SPII): 0.626961
- Cleavage Site: CS pos: 15-16. GAT-CA. Pr: 0.6087
- predicted transmembrane helices (TMHMM): 0
⊟Accession numbers[edit | edit source]
⊟Protein sequence[edit | edit source]
- MPKIVVVGAVAGGATCASQIRRLDKESDIIIFEKDRDMSFANCALPYVIGEVVEDRRYALAYTPEEFYDRKQITVKTYHEVIAINDERQTVSVLNRKTNEQFEESYDKLILSPGASANSLGFESDITFTLRNLEDTDAIDQFIKANQVDKVLVVGAGYVSLEVLENLYERGLHPTLIHRSDKINKLMDADMNQPILDELDKREIPYRLNEEINAINGNEITFKSGKVEHYDMIIEGVGTHPNSKFIESSNIKLDRKGFIPVNDKFETNVPNIYAIGDIATSHYRHVDLPASVPLAWGAHRAASIVAEQIAGNDTIEFKGFLGNNIVKFFDYTFASVGVKPNELKQFDYKMVEVTQGAHANYYPGNSPLHLRVYYDTSNRQILRAAAVGKEGADKRIDVLSMAMMNQLTVDELTEFEVAYAPPYSHPKDLINMIGYKAK
⊟Experimental data[edit | edit source]
⊟Expression & Regulation[edit | edit source]
⊟Operon[edit | edit source]
⊟Regulation[edit | edit source]
- regulator:
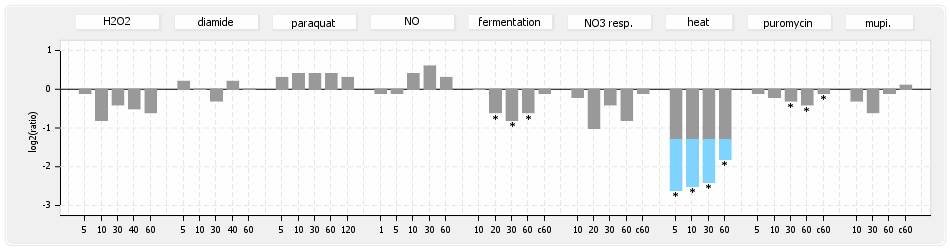
⊟Transcription pattern[edit | edit source]
- S.aureus Expression Data Browser: data available for NCTC8325
⊟Protein synthesis (provided by Aureolib)[edit | edit source]
⊟Protein stability[edit | edit source]
- half-life: 27.73 h [6]
⊟Biological Material[edit | edit source]
⊟Mutants[edit | edit source]
⊟Expression vector[edit | edit source]
⊟lacZ fusion[edit | edit source]
⊟GFP fusion[edit | edit source]
⊟two-hybrid system[edit | edit source]
⊟FLAG-tag construct[edit | edit source]
⊟Antibody[edit | edit source]
⊟Other Information[edit | edit source]
You can add further information about the gene and protein here. [edit]
⊟Literature[edit | edit source]
⊟References[edit | edit source]
- ↑ Dörte Becher, Kristina Hempel, Susanne Sievers, Daniela Zühlke, Jan Pané-Farré, Andreas Otto, Stephan Fuchs, Dirk Albrecht, Jörg Bernhardt, Susanne Engelmann, Uwe Völker, Jan Maarten van Dijl, Michael Hecker
A proteomic view of an important human pathogen--towards the quantification of the entire Staphylococcus aureus proteome.
PLoS One: 2009, 4(12);e8176
[PubMed:19997597] [WorldCat.org] [DOI] (I e) - ↑ Kristina Hempel, Jan Pané-Farré, Andreas Otto, Susanne Sievers, Michael Hecker, Dörte Becher
Quantitative cell surface proteome profiling for SigB-dependent protein expression in the human pathogen Staphylococcus aureus via biotinylation approach.
J Proteome Res: 2010, 9(3);1579-90
[PubMed:20108986] [WorldCat.org] [DOI] (I p) - ↑ Kristina Hempel, Florian-Alexander Herbst, Martin Moche, Michael Hecker, Dörte Becher
Quantitative proteomic view on secreted, cell surface-associated, and cytoplasmic proteins of the methicillin-resistant human pathogen Staphylococcus aureus under iron-limited conditions.
J Proteome Res: 2011, 10(4);1657-66
[PubMed:21323324] [WorldCat.org] [DOI] (I p) - ↑ Andreas Otto, Jan Maarten van Dijl, Michael Hecker, Dörte Becher
The Staphylococcus aureus proteome.
Int J Med Microbiol: 2014, 304(2);110-20
[PubMed:24439828] [WorldCat.org] [DOI] (I p) - ↑ Daniela Zühlke, Kirsten Dörries, Jörg Bernhardt, Sandra Maaß, Jan Muntel, Volkmar Liebscher, Jan Pané-Farré, Katharina Riedel, Michael Lalk, Uwe Völker, Susanne Engelmann, Dörte Becher, Stephan Fuchs, Michael Hecker
Costs of life - Dynamics of the protein inventory of Staphylococcus aureus during anaerobiosis.
Sci Rep: 2016, 6;28172
[PubMed:27344979] [WorldCat.org] [DOI] (I e) - ↑ Stephan Michalik, Jörg Bernhardt, Andreas Otto, Martin Moche, Dörte Becher, Hanna Meyer, Michael Lalk, Claudia Schurmann, Rabea Schlüter, Holger Kock, Ulf Gerth, Michael Hecker
Life and death of proteins: a case study of glucose-starved Staphylococcus aureus.
Mol Cell Proteomics: 2012, 11(9);558-70
[PubMed:22556279] [WorldCat.org] [DOI] (I p)