Jump to navigation
Jump to search
NCBI: 10-JUN-2013
⊟Summary[edit | edit source]
- organism: Staphylococcus aureus COL
- locus tag: SACOL1104 [new locus tag: SACOL_RS05640 ]
- pan locus tag?: SAUPAN003319000
- symbol: pdhC
- pan gene symbol?: pdhC
- synonym:
- product: branched-chain alpha-keto acid dehydrogenase E2
⊟Genome View[edit | edit source]
⊟Gene[edit | edit source]
⊟General[edit | edit source]
- type: CDS
- locus tag: SACOL1104 [new locus tag: SACOL_RS05640 ]
- symbol: pdhC
- product: branched-chain alpha-keto acid dehydrogenase E2
- replicon: chromosome
- strand: +
- coordinates: 1114254..1115546
- length: 1293
- essential: unknown other strains
⊟Accession numbers[edit | edit source]
- Gene ID: 3237847 NCBI
- RefSeq: YP_185968 NCBI
- BioCyc: see SACOL_RS05640
- MicrobesOnline: 912572 MicrobesOnline
⊟Phenotype[edit | edit source]
Share your knowledge and add information here. [edit]
⊟DNA sequence[edit | edit source]
- 1
61
121
181
241
301
361
421
481
541
601
661
721
781
841
901
961
1021
1081
1141
1201
1261GTGGCATTTGAATTTAGATTACCCGATATCGGGGAAGGTATCCACGAAGGTGAAATTGTA
AAATGGTTTGTTAAAGCTGGAGATACTATTGAAGAAGACGATGTTTTAGCTGAGGTACAA
AACGATAAATCAGTAGTAGAAATCCCATCACCAGTATCTGGTACTGTAGAAGAAGTTATG
GTAGAAGAAGGTACAGTAGCTGTAGTTGGTGACGTTATTGTTAAAATCGATGCACCTGAT
GCAGAAGATATGCAATTTAAAGGTCATGATGATGATTCATCATCTAAAGAAGAACCTGCG
AAAGAGGAAGCGCCAGCAGAGCAAGCACCTGTAGCTACTCAAACTGAAGAAGTAGATGAA
AACAGAACTGTTAAAGCAATGCCTTCAGTACGTAAATACGCACGTGAAAAAGGTGTTAAC
ATTAAAGCAGTTTCTGGATCTGGTAAAAATGGTCGTATTACAAAAGAAGATGTAGATGCA
TACTTAAATGGTGGTGCACCAACAGCTTCAAATGAATCAGCTGCTTCAGCTACAAGTGAA
GAAGTTGCTGAAACTCCTGCAGCACCTGCAGCAGTAACATTAGAAGGCGACTTCCCAGAA
ACAACTGAAAAAATCCCTGCTATGCGTAGAGCAATTGCGAAAGCAATGGTTAACTCTAAG
CATACTGCACCTCATGTAACATTAATGGATGAAATTGATGTTCAAGCATTATGGGATCAC
CGTAAGAAATTTAAAGAAATCGCAGCTGAACAAGGTACTAAGTTAACATTCTTACCTTAT
GTTGTTAAAGCACTTGTTTCTGCATTGAAAAAATACCCAGCACTTAACACTTCATTCAAT
GAAGAAGCTGGTGAAATCGTTCATAAACATTACTGGAATATCGGTATTGCAGCAGACACT
GATAGAGGATTATTAGTACCTGTTGTTAAACATGCTGATCGTAAGTCTATTTTCCAAATT
TCAGATGAAATTAATGAATTAGCTGTTAAAGCACGTGATGGTAAATTAACAGCCGATGAA
ATGAAAGGTGCTACATGCACAATCAGTAATATCGGTTCAGCTGGTGGACAATGGTTCACT
CCAGTTATCAATCACCCAGAAGTAGCAATCTTAGGAATTGGCCGTATTGCTCAAAAACCT
ATCGTTAAAGATGGAGAAATTGTTGCAGCACCAGTATTAGCATTATCATTAAGCTTTGAC
CACAGACAAATTGATGGTGCAACTGGCCAAAATGCAATGAATCACATTAAACGTTTATTA
AATAATCCAGAATTATTATTAATGGAGGGGTAA60
120
180
240
300
360
420
480
540
600
660
720
780
840
900
960
1020
1080
1140
1200
1260
1293
⊟Protein[edit | edit source]
⊟General[edit | edit source]
- locus tag: SACOL1104 [new locus tag: SACOL_RS05640 ]
- symbol: PdhC
- description: branched-chain alpha-keto acid dehydrogenase E2
- length: 430
- theoretical pI: 4.63498
- theoretical MW: 46382
- GRAVY: -0.292093
⊟Function[edit | edit source]
- reaction: EC 2.3.1.12? ExPASyDihydrolipoyllysine-residue acetyltransferase Acetyl-CoA + enzyme N6-(dihydrolipoyl)lysine = CoA + enzyme N6-(S-acetyldihydrolipoyl)lysine
- TIGRFAM: Energy metabolism Pyruvate dehydrogenase dihydrolipoyllysine-residue acetyltransferase (TIGR01348; EC 2.3.1.12; HMM-score: 359)Energy metabolism TCA cycle dihydrolipoyllysine-residue succinyltransferase, E2 component of oxoglutarate dehydrogenase (succinyl-transferring) complex (TIGR01347; EC 2.3.1.61; HMM-score: 310.9)2-oxoglutarate dehydrogenase, E2 component, dihydrolipoamide succinyltransferase (TIGR02927; EC 2.3.1.61; HMM-score: 296.2)and 12 moreEnergy metabolism Pyruvate dehydrogenase pyruvate dehydrogenase complex dihydrolipoamide acetyltransferase (TIGR01349; EC 2.3.1.12; HMM-score: 274.9)Transport and binding proteins Other efflux pump membrane protein (TIGR00998; HMM-score: 22.3)Transport and binding proteins Cations and iron carrying compounds oxaloacetate decarboxylase alpha subunit (TIGR01108; EC 4.1.1.3; HMM-score: 18.4)Energy metabolism Other oxaloacetate decarboxylase alpha subunit (TIGR01108; EC 4.1.1.3; HMM-score: 18.4)Fatty acid and phospholipid metabolism Biosynthesis acetyl-CoA carboxylase, biotin carboxyl carrier protein (TIGR00531; HMM-score: 17.3)Central intermediary metabolism Nitrogen metabolism urea carboxylase (TIGR02712; EC 6.3.4.6; HMM-score: 17.3)Transport and binding proteins Unknown substrate efflux transporter, RND family, MFP subunit (TIGR01730; HMM-score: 16.5)Energy metabolism Amino acids and amines glycine cleavage system H protein (TIGR00527; HMM-score: 15.7)Energy metabolism Glycolysis/gluconeogenesis pyruvate carboxylase (TIGR01235; EC 6.4.1.1; HMM-score: 15.5)glycine cleavage protein H-like protein (TIGR03077; HMM-score: 14.9)Cellular processes Biosynthesis of natural products NHLM bacteriocin system secretion protein (TIGR03794; HMM-score: 12.2)Transport and binding proteins Amino acids, peptides and amines NHLM bacteriocin system secretion protein (TIGR03794; HMM-score: 12.2)
- TheSEED :
- Dihydrolipoamide acetyltransferase component of pyruvate dehydrogenase complex (EC 2.3.1.12)
Carbohydrates Central carbohydrate metabolism Dehydrogenase complexes Dihydrolipoamide acetyltransferase component of pyruvate dehydrogenase complex (EC 2.3.1.12)and 2 more - PFAM: CoA-acyltrans (CL0149) 2-oxoacid_dh; 2-oxoacid dehydrogenases acyltransferase (catalytic domain) (PF00198; HMM-score: 313.3)and 12 moreHybrid (CL0105) Biotin_lipoyl; Biotin-requiring enzyme (PF00364; HMM-score: 84.2)no clan defined E3_binding; e3 binding domain (PF02817; HMM-score: 63)Hybrid (CL0105) Biotin_lipoyl_2; Biotin-lipoyl like (PF13533; HMM-score: 43.5)GCV_H; Glycine cleavage H-protein (PF01597; HMM-score: 19.4)ATP-synt_ab_Xtn; ATPsynthase alpha/beta subunit barrel-sandwich domain (PF16886; HMM-score: 17.9)NQRA_N; NqrA N-terminal barrel-sandwich hybrid domain (PF05896; HMM-score: 15.3)RnfC_N; RnfC Barrel sandwich hybrid domain (PF13375; HMM-score: 15.3)HlyD_D23; Barrel-sandwich domain of CusB or HlyD membrane-fusion (PF16576; HMM-score: 14.1)no clan defined TMP_3; Tape measure protein (PF20155; HMM-score: 13.6)DUF896; Bacterial protein of unknown function (DUF896) (PF05979; HMM-score: 13)TIM_barrel (CL0036) DUF2090; Uncharacterized protein conserved in bacteria (DUF2090) (PF09863; HMM-score: 11.8)Hybrid (CL0105) HlyD_3; HlyD family secretion protein (PF13437; HMM-score: 11.5)
⊟Structure, modifications & cofactors[edit | edit source]
- domains:
- modifications:
- cofactors: (R)-lipoate
- effectors:
⊟Localization[edit | edit source]
- PSORTb: Cytoplasmic
- Cytoplasmic Score: 9.97
- Cytoplasmic Membrane Score: 0
- Cellwall Score: 0.01
- Extracellular Score: 0.02
- Internal Helices: 0
- DeepLocPro: Cytoplasmic
- Cytoplasmic Score: 0.9941
- Cytoplasmic Membrane Score: 0.0041
- Cell wall & surface Score: 0
- Extracellular Score: 0.0018
- LocateP: Intracellular
- Prediction by SwissProt Classification: Cytoplasmic
- Pathway Prediction: No pathway
- Intracellular possibility: 1
- Signal peptide possibility: -1
- N-terminally Anchored Score: 1
- Predicted Cleavage Site: No CleavageSite
- SignalP: no predicted signal peptide
- SP(Sec/SPI): 0.002784
- TAT(Tat/SPI): 0.000262
- LIPO(Sec/SPII): 0.000333
- predicted transmembrane helices (TMHMM): 0
⊟Accession numbers[edit | edit source]
⊟Protein sequence[edit | edit source]
- MAFEFRLPDIGEGIHEGEIVKWFVKAGDTIEEDDVLAEVQNDKSVVEIPSPVSGTVEEVMVEEGTVAVVGDVIVKIDAPDAEDMQFKGHDDDSSSKEEPAKEEAPAEQAPVATQTEEVDENRTVKAMPSVRKYAREKGVNIKAVSGSGKNGRITKEDVDAYLNGGAPTASNESAASATSEEVAETPAAPAAVTLEGDFPETTEKIPAMRRAIAKAMVNSKHTAPHVTLMDEIDVQALWDHRKKFKEIAAEQGTKLTFLPYVVKALVSALKKYPALNTSFNEEAGEIVHKHYWNIGIAADTDRGLLVPVVKHADRKSIFQISDEINELAVKARDGKLTADEMKGATCTISNIGSAGGQWFTPVINHPEVAILGIGRIAQKPIVKDGEIVAAPVLALSLSFDHRQIDGATGQNAMNHIKRLLNNPELLLMEG
⊟Experimental data[edit | edit source]
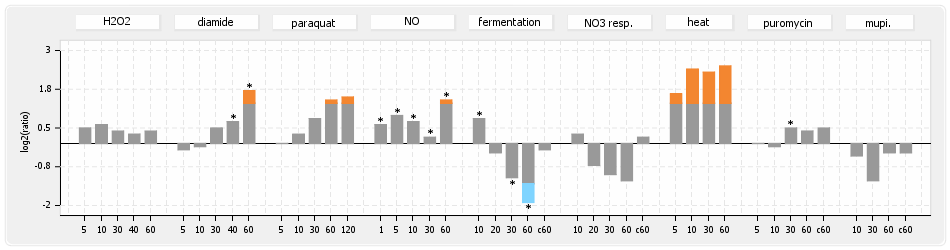
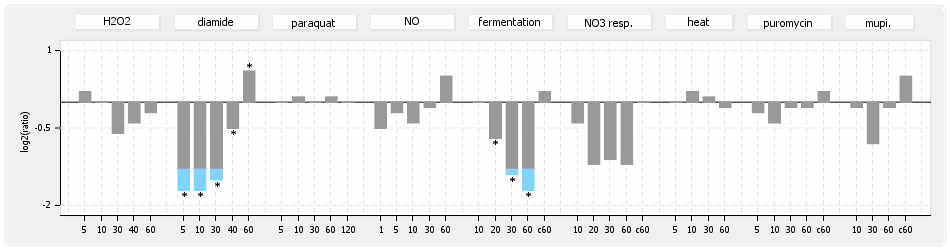
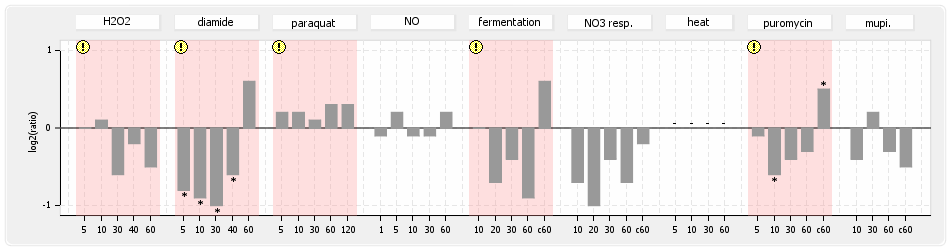
⊟Expression & Regulation[edit | edit source]
⊟Operon[edit | edit source]
- MicrobesOnline: pdhA > pdhB > pdhC > pdhD
⊟Regulation[edit | edit source]
- regulator:
⊟Transcription pattern[edit | edit source]
- S.aureus Expression Data Browser: data available for NCTC8325
⊟Protein synthesis (provided by Aureolib)[edit | edit source]
⊟Protein stability[edit | edit source]
- half-life: 18.99 h [6]
⊟Biological Material[edit | edit source]
⊟Mutants[edit | edit source]
⊟Expression vector[edit | edit source]
⊟lacZ fusion[edit | edit source]
⊟GFP fusion[edit | edit source]
⊟two-hybrid system[edit | edit source]
⊟FLAG-tag construct[edit | edit source]
⊟Antibody[edit | edit source]
⊟Other Information[edit | edit source]
You can add further information about the gene and protein here. [edit]
⊟Literature[edit | edit source]
⊟References[edit | edit source]
- ↑ Dörte Becher, Kristina Hempel, Susanne Sievers, Daniela Zühlke, Jan Pané-Farré, Andreas Otto, Stephan Fuchs, Dirk Albrecht, Jörg Bernhardt, Susanne Engelmann, Uwe Völker, Jan Maarten van Dijl, Michael Hecker
A proteomic view of an important human pathogen--towards the quantification of the entire Staphylococcus aureus proteome.
PLoS One: 2009, 4(12);e8176
[PubMed:19997597] [WorldCat.org] [DOI] (I e) - ↑ Kristina Hempel, Jan Pané-Farré, Andreas Otto, Susanne Sievers, Michael Hecker, Dörte Becher
Quantitative cell surface proteome profiling for SigB-dependent protein expression in the human pathogen Staphylococcus aureus via biotinylation approach.
J Proteome Res: 2010, 9(3);1579-90
[PubMed:20108986] [WorldCat.org] [DOI] (I p) - ↑ Kristina Hempel, Florian-Alexander Herbst, Martin Moche, Michael Hecker, Dörte Becher
Quantitative proteomic view on secreted, cell surface-associated, and cytoplasmic proteins of the methicillin-resistant human pathogen Staphylococcus aureus under iron-limited conditions.
J Proteome Res: 2011, 10(4);1657-66
[PubMed:21323324] [WorldCat.org] [DOI] (I p) - ↑ Andreas Otto, Jan Maarten van Dijl, Michael Hecker, Dörte Becher
The Staphylococcus aureus proteome.
Int J Med Microbiol: 2014, 304(2);110-20
[PubMed:24439828] [WorldCat.org] [DOI] (I p) - ↑ Daniela Zühlke, Kirsten Dörries, Jörg Bernhardt, Sandra Maaß, Jan Muntel, Volkmar Liebscher, Jan Pané-Farré, Katharina Riedel, Michael Lalk, Uwe Völker, Susanne Engelmann, Dörte Becher, Stephan Fuchs, Michael Hecker
Costs of life - Dynamics of the protein inventory of Staphylococcus aureus during anaerobiosis.
Sci Rep: 2016, 6;28172
[PubMed:27344979] [WorldCat.org] [DOI] (I e) - ↑ Stephan Michalik, Jörg Bernhardt, Andreas Otto, Martin Moche, Dörte Becher, Hanna Meyer, Michael Lalk, Claudia Schurmann, Rabea Schlüter, Holger Kock, Ulf Gerth, Michael Hecker
Life and death of proteins: a case study of glucose-starved Staphylococcus aureus.
Mol Cell Proteomics: 2012, 11(9);558-70
[PubMed:22556279] [WorldCat.org] [DOI] (I p)