Jump to navigation
Jump to search
NCBI: 10-JUN-2013
⊟Summary[edit | edit source]
- organism: Staphylococcus aureus COL
- locus tag: SACOL1965 [new locus tag: SACOL_RS10275 ]
- pan locus tag?: SAUPAN004933000
- symbol: ligA
- pan gene symbol?: ligA
- synonym:
- product: DNA ligase, NAD-dependent
⊟Genome View[edit | edit source]
⊟Gene[edit | edit source]
⊟General[edit | edit source]
- type: CDS
- locus tag: SACOL1965 [new locus tag: SACOL_RS10275 ]
- symbol: ligA
- product: DNA ligase, NAD-dependent
- replicon: chromosome
- strand: -
- coordinates: 2026692..2028695
- length: 2004
- essential: unknown other strains
⊟Accession numbers[edit | edit source]
- Gene ID: 3238150 NCBI
- RefSeq: YP_186789 NCBI
- BioCyc: see SACOL_RS10275
- MicrobesOnline: 913443 MicrobesOnline
⊟Phenotype[edit | edit source]
Share your knowledge and add information here. [edit]
⊟DNA sequence[edit | edit source]
- 1
61
121
181
241
301
361
421
481
541
601
661
721
781
841
901
961
1021
1081
1141
1201
1261
1321
1381
1441
1501
1561
1621
1681
1741
1801
1861
1921
1981ATGGCTGATTTATCGTCTCGTGTGAACGAGTTACATGATTTATTAAATCAATACAGTTAT
GAATACTATGTAGAGGATAATCCATCTGTACCAGATAGTGAATATGACAAATTACTTCAT
GAACTGATTAAAATAGAAGAGGAGCATCCTGAGTATAAGACTGTAGATTCTCCAACAGTT
AGAGTTGGCGGTGAAGCCCAAGCCTCTTTCAATAAAGTCAACCATGACACGCCAATGTTA
AGTTTAGGGAATGCATTTAATGAGGATGATTTGAGAAAATTCGACCAACGCATACGTGAA
CAAATTGGCAACGTTGAATATATGTGCGAATTAAAAATTGATGGCTTAGCAGTATCATTG
AAATATGTTGATGGATACTTCGTTCAAGGTTTAACACGTGGTGATGGAACAACAGGTGAA
GATATTACCGAAAATTTAAAAACAATTCATGCGATACCTTTGAAAATGAAAGAACCATTA
AATGTAGAAGTTCGTGGTGAAGCATATATGCCGAGACGTTCATTTTTACGATTAAATGAA
GAAAAAGAAAAAAATGATGAGCAGTTATTTGCAAATCCAAGAAACGCTGCTGCGGGATCA
TTAAGACAGTTAGATTCTAAATTAACGGCAAAACGAAAGCTAAGCGTATTTATATATAGT
GTCAATGATTTCACTGATTTCAATGCGCGTTCGCAAAGTGAAGCATTAGATGAGTTAAAT
AAATTAGGTTTTACAACGAATAAAAATAGAGCGCGTGTAAATAATATCGATGGTGTTTTA
GAGTATATTGAAAAATGGACAAGCCAAAGAGAGTCATTACCTTATGATATTGATGGGATT
GTTATTAAGGTTAATGATTTAGATCAACAGGATGAGATGGGATTCACACAAAAATCTCCT
AGATGGGCCATTGCTTATAAATTTCCAGCTGAGGAAGTAGTAACTAAATTATTAGATATT
GAATTAAGTATTGGACGAACAGGTGTAGTCACACCTACTGCTATTTTAGAACCAGTAAAA
GTAGCTGGTACAACTGTATCAAGAGCATCTTTGCACAATGAGGATTTAATTCATGACAGA
GATATTCGAATTGGTGATAGTGTTGTAGTGAAAAAAGCAGGTGACATCATACCTGAAGTT
GTACGTAGTATTCCAGAACGTAGACCTGAGGATGCTGTCACATATCATATGCCAACCCAT
TGTCCAAGTTGTGGACATGAATTAGTACGTATTGAAGGCGAAGTAGCACTTCGTTGCATT
AATCCAAAATGCCAAGCACAACTTGTTGAAGGATTGATTCACTTTGTATCAAGACAAGCC
ATGAATATTGATGGTTTAGGCACTAAAATTATTCAACAGCTTTATCAAAGCGAATTAATT
AAAGATGTTGCTGATATTTTCTATTTAACAGAAGAAGATTTATTACCTTTAGACAGAATG
GGGCAGAAAAAAGTTGATAATTTATTAGCTGCCATTCAACAAGCTAAGGACAACTCTTTA
GAAAATTTATTATTTGGTCTAGGTATTAGGCATTTAGGTGTTAAAGCGAGCCAAGTGTTA
GCAGAAAAATATGAAACGATAGATCGATTACTAACGGTAACTGAAGCGGAATTAGTAGAA
ATTCATGATATAGGTGATAAAGTAGCACAATCTGTAGTTACTTATTTAGAAAATGAAGAT
ATTCGTGCTTTAATTCAAAAATTAAAAGATAAACATGTTAATATGATTTATAAAGGTATC
AAAACATCAGATATTGAAGGACATCCTGAATTTAGTGGTAAAACGATAGTACTGACTGGT
AAGCTACATCAAATGACACGCAATGAAGCATCTAAATGGCTTGCATCACAAGGTGCTAAA
GTTACAAGTAGCGTTACTAAAAATACAGATGTCGTTATTGCTGGTGAAGATGCAGGTTCA
AAATTAACAAAAGCACAAAGTTTAGGTATTGAAATTTGGACAGAGCAACAATTTGTAGAT
AAGCAAAATGAATTAAATAGTTAG60
120
180
240
300
360
420
480
540
600
660
720
780
840
900
960
1020
1080
1140
1200
1260
1320
1380
1440
1500
1560
1620
1680
1740
1800
1860
1920
1980
2004
⊟Protein[edit | edit source]
⊟General[edit | edit source]
- locus tag: SACOL1965 [new locus tag: SACOL_RS10275 ]
- symbol: LigA
- description: DNA ligase, NAD-dependent
- length: 667
- theoretical pI: 4.94308
- theoretical MW: 75079.5
- GRAVY: -0.426087
⊟Function[edit | edit source]
- reaction: EC 6.5.1.2? ExPASyDNA ligase (NAD+) NAD+ + (deoxyribonucleotide)(n)-3'-hydroxyl + 5'-phospho-(deoxyribonucleotide)(m) = (deoxyribonucleotide)(n+m) + AMP + beta-nicotinamide D-nucleotide
- TIGRFAM: DNA metabolism DNA replication, recombination, and repair DNA ligase, NAD-dependent (TIGR00575; EC 6.5.1.2; HMM-score: 880.9)and 2 moreTranscription DNA-dependent RNA polymerase DNA-directed RNA polymerase, alpha subunit (TIGR02027; EC 2.7.7.6; HMM-score: 13.8)Protein fate Degradation of proteins, peptides, and glycopeptides zinc-dependent metalloproteinase lipoprotein, BF0631 family (TIGR03952; EC 3.4.24.-; HMM-score: 12.2)
- TheSEED :
- DNA ligase (EC 6.5.1.2)
- PFAM: ATP-grasp (CL0179) DNA_ligase_aden; NAD-dependent DNA ligase adenylation domain (PF01653; HMM-score: 344.3)and 13 moreOB (CL0021) DNA_ligase_OB; NAD-dependent DNA ligase OB-fold domain (PF03120; HMM-score: 116.9)HHH (CL0198) HHH_2; Helix-hairpin-helix motif (PF12826; HMM-score: 74.5)BRCT-like (CL0459) BRCT; BRCA1 C Terminus (BRCT) domain (PF00533; HMM-score: 61.8)no clan defined DNA_ligase_ZBD; NAD-dependent DNA ligase C4 zinc finger domain (PF03119; HMM-score: 47.6)HHH (CL0198) HHH_5; Helix-hairpin-helix domain (PF14520; HMM-score: 38.8)no clan defined Nlig-Ia; DNA ligase-like, N-terminal NAD+-binding domain (PF22745; HMM-score: 26.5)BRCT-like (CL0459) PTCB-BRCT; twin BRCT domain (PF12738; HMM-score: 21.9)HHH (CL0198) RNA_pol_A_CTD; Bacterial RNA polymerase, alpha chain C terminal domain (PF03118; HMM-score: 21.6)Zn_Beta_Ribbon (CL0167) Zn_ribbon_NUD; NADH pyrophosphatase zinc ribbon domain (PF09297; HMM-score: 21.6)HHH (CL0198) HHH; Helix-hairpin-helix motif (PF00633; HMM-score: 14.8)ATP-grasp (CL0179) RNA_ligase; RNA ligase (PF09414; HMM-score: 14.8)no clan defined zf-IS66; zinc-finger binding domain of transposase IS66 (PF13005; HMM-score: 14.2)Zn_Beta_Ribbon (CL0167) DUF7575; Domain of unknown function (DUF7575) (PF24460; HMM-score: 11.1)
⊟Structure, modifications & cofactors[edit | edit source]
- domains:
- modifications:
- cofactors: Mg2+, Mn2+
- effectors:
⊟Localization[edit | edit source]
- PSORTb: Cytoplasmic
- Cytoplasmic Score: 7.5
- Cytoplasmic Membrane Score: 1.15
- Cellwall Score: 0.62
- Extracellular Score: 0.73
- Internal Helices: 0
- DeepLocPro: Cytoplasmic
- Cytoplasmic Score: 0.999
- Cytoplasmic Membrane Score: 0.0003
- Cell wall & surface Score: 0.0001
- Extracellular Score: 0.0006
- LocateP: Intracellular
- Prediction by SwissProt Classification: Cytoplasmic
- Pathway Prediction: No pathway
- Intracellular possibility: 1
- Signal peptide possibility: -1
- N-terminally Anchored Score: -1
- Predicted Cleavage Site: No CleavageSite
- SignalP: no predicted signal peptide
- SP(Sec/SPI): 0.003115
- TAT(Tat/SPI): 0.000545
- LIPO(Sec/SPII): 0.000491
- predicted transmembrane helices (TMHMM): 0
⊟Accession numbers[edit | edit source]
⊟Protein sequence[edit | edit source]
- MADLSSRVNELHDLLNQYSYEYYVEDNPSVPDSEYDKLLHELIKIEEEHPEYKTVDSPTVRVGGEAQASFNKVNHDTPMLSLGNAFNEDDLRKFDQRIREQIGNVEYMCELKIDGLAVSLKYVDGYFVQGLTRGDGTTGEDITENLKTIHAIPLKMKEPLNVEVRGEAYMPRRSFLRLNEEKEKNDEQLFANPRNAAAGSLRQLDSKLTAKRKLSVFIYSVNDFTDFNARSQSEALDELNKLGFTTNKNRARVNNIDGVLEYIEKWTSQRESLPYDIDGIVIKVNDLDQQDEMGFTQKSPRWAIAYKFPAEEVVTKLLDIELSIGRTGVVTPTAILEPVKVAGTTVSRASLHNEDLIHDRDIRIGDSVVVKKAGDIIPEVVRSIPERRPEDAVTYHMPTHCPSCGHELVRIEGEVALRCINPKCQAQLVEGLIHFVSRQAMNIDGLGTKIIQQLYQSELIKDVADIFYLTEEDLLPLDRMGQKKVDNLLAAIQQAKDNSLENLLFGLGIRHLGVKASQVLAEKYETIDRLLTVTEAELVEIHDIGDKVAQSVVTYLENEDIRALIQKLKDKHVNMIYKGIKTSDIEGHPEFSGKTIVLTGKLHQMTRNEASKWLASQGAKVTSSVTKNTDVVIAGEDAGSKLTKAQSLGIEIWTEQQFVDKQNELNS
⊟Experimental data[edit | edit source]
- experimentally validated: PeptideAtlas
- protein localization: Cytoplasmic [1] [2] [3]
- quantitative data / protein copy number per cell: 233 [4]
- interaction partners:
SACOL1760 (ackA) acetate kinase [5] (data from MRSA252) SACOL0937 (dltC) D-alanine--poly(phosphoribitol) ligase subunit 2 [5] (data from MRSA252) SACOL1199 (ftsZ) cell division protein FtsZ [5] (data from MRSA252) SACOL0593 (fusA) elongation factor G [5] (data from MRSA252) SACOL0006 (gyrA) DNA gyrase, A subunit [5] (data from MRSA252) SACOL1513 (hup) DNA-binding protein HU [5] (data from MRSA252) SACOL1741 (icd) isocitrate dehydrogenase [5] (data from MRSA252) SACOL2623 (mqo2) malate:quinone oxidoreductase [5] (data from MRSA252) SACOL1102 (pdhA) pyruvate dehydrogenase complex E1 component subunit alpha [5] (data from MRSA252) SACOL1103 (pdhB) pyruvate dehydrogenase complex E1 component subunit beta [5] (data from MRSA252) SACOL1104 (pdhC) branched-chain alpha-keto acid dehydrogenase E2 [5] (data from MRSA252) SACOL1105 (pdhD) dihydrolipoamide dehydrogenase [5] (data from MRSA252) SACOL0204 (pflB) formate acetyltransferase [5] (data from MRSA252) SACOL1737 (polA) DNA polymerase I [5] (data from MRSA252) SACOL1745 (pyk) pyruvate kinase [5] (data from MRSA252) SACOL1812 (rot) repressor of toxins [5] (data from MRSA252) SACOL0584 (rplA) 50S ribosomal protein L1 [5] (data from MRSA252) SACOL2236 (rplB) 50S ribosomal protein L2 [5] (data from MRSA252) SACOL2239 (rplC) 50S ribosomal protein L3 [5] (data from MRSA252) SACOL2224 (rplF) 50S ribosomal protein L6 [5] (data from MRSA252) SACOL0585 (rplJ) 50S ribosomal protein L10 [5] (data from MRSA252) SACOL0586 (rplL) 50S ribosomal protein L7/L12 [5] (data from MRSA252) SACOL2220 (rplO) 50S ribosomal protein L15 [5] (data from MRSA252) SACOL2212 (rplQ) 50S ribosomal protein L17 [5] (data from MRSA252) SACOL1257 (rplS) 50S ribosomal protein L19 [5] (data from MRSA252) SACOL1702 (rplU) 50S ribosomal protein L21 [5] (data from MRSA252) SACOL2234 (rplV) 50S ribosomal protein L22 [5] (data from MRSA252) SACOL2213 (rpoA) DNA-directed RNA polymerase subunit alpha [5] (data from MRSA252) SACOL0588 (rpoB) DNA-directed RNA polymerase subunit beta [5] (data from MRSA252) SACOL0589 (rpoC) DNA-directed RNA polymerase subunit beta' [5] (data from MRSA252) SACOL2222 (rpsE) 30S ribosomal protein S5 [5] (data from MRSA252) SACOL2206 (rpsI) 30S ribosomal protein S9 [5] (data from MRSA252) SACOL2214 (rpsK) 30S ribosomal protein S11 [5] (data from MRSA252) SACOL1292 (rpsO) 30S ribosomal protein S15 [5] (data from MRSA252) SACOL2230 (rpsQ) 30S ribosomal protein S17 [5] (data from MRSA252) SACOL2287 (sarR) accessory regulator R [5] (data from MRSA252) SACOL0095 (spa) immunoglobulin G binding protein A precursor [5] (data from MRSA252) SACOL0541 (spoVG) regulatory protein SpoVG [5] (data from MRSA252) SACOL1449 (sucA) 2-oxoglutarate dehydrogenase E1 component [5] (data from MRSA252) SACOL1448 (sucB) dihydrolipoamide succinyltransferase [5] (data from MRSA252) SACOL1267 (topA) DNA topoisomerase I [5] (data from MRSA252) SACOL1276 (tsf) elongation factor Ts [5] (data from MRSA252) SACOL0594 (tuf) elongation factor Tu [5] (data from MRSA252) SACOL0212 3-hydroxyacyl-CoA dehydrogenase [5] (data from MRSA252) SACOL0731 LysR family transcriptional regulator [5] (data from MRSA252) SACOL0944 NADH dehydrogenase [5] (data from MRSA252) SACOL1753 universal stress protein [5] (data from MRSA252) SACOL2561 hydroxymethylglutaryl-CoA synthase [5] (data from MRSA252)
⊟Expression & Regulation[edit | edit source]
⊟Operon[edit | edit source]
- MicrobesOnline: camS < ligA < pcrA < SACOL1967
⊟Regulation[edit | edit source]
- regulator:
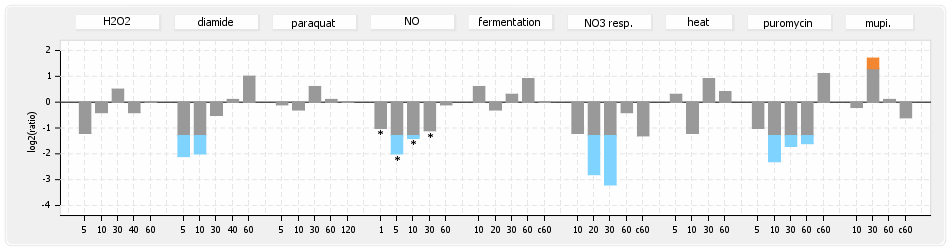
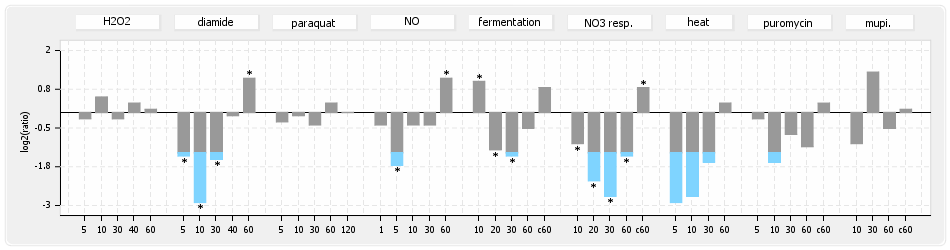
⊟Transcription pattern[edit | edit source]
- S.aureus Expression Data Browser: data available for NCTC8325
⊟Protein synthesis (provided by Aureolib)[edit | edit source]
⊟Protein stability[edit | edit source]
- half-life: 36.22 h [6]
⊟Biological Material[edit | edit source]
⊟Mutants[edit | edit source]
⊟Expression vector[edit | edit source]
⊟lacZ fusion[edit | edit source]
⊟GFP fusion[edit | edit source]
⊟two-hybrid system[edit | edit source]
⊟FLAG-tag construct[edit | edit source]
⊟Antibody[edit | edit source]
⊟Other Information[edit | edit source]
You can add further information about the gene and protein here. [edit]
⊟Literature[edit | edit source]
⊟References[edit | edit source]
- ↑ Dörte Becher, Kristina Hempel, Susanne Sievers, Daniela Zühlke, Jan Pané-Farré, Andreas Otto, Stephan Fuchs, Dirk Albrecht, Jörg Bernhardt, Susanne Engelmann, Uwe Völker, Jan Maarten van Dijl, Michael Hecker
A proteomic view of an important human pathogen--towards the quantification of the entire Staphylococcus aureus proteome.
PLoS One: 2009, 4(12);e8176
[PubMed:19997597] [WorldCat.org] [DOI] (I e) - ↑ Kristina Hempel, Florian-Alexander Herbst, Martin Moche, Michael Hecker, Dörte Becher
Quantitative proteomic view on secreted, cell surface-associated, and cytoplasmic proteins of the methicillin-resistant human pathogen Staphylococcus aureus under iron-limited conditions.
J Proteome Res: 2011, 10(4);1657-66
[PubMed:21323324] [WorldCat.org] [DOI] (I p) - ↑ Andreas Otto, Jan Maarten van Dijl, Michael Hecker, Dörte Becher
The Staphylococcus aureus proteome.
Int J Med Microbiol: 2014, 304(2);110-20
[PubMed:24439828] [WorldCat.org] [DOI] (I p) - ↑ Daniela Zühlke, Kirsten Dörries, Jörg Bernhardt, Sandra Maaß, Jan Muntel, Volkmar Liebscher, Jan Pané-Farré, Katharina Riedel, Michael Lalk, Uwe Völker, Susanne Engelmann, Dörte Becher, Stephan Fuchs, Michael Hecker
Costs of life - Dynamics of the protein inventory of Staphylococcus aureus during anaerobiosis.
Sci Rep: 2016, 6;28172
[PubMed:27344979] [WorldCat.org] [DOI] (I e) - ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 5.17 5.18 5.19 5.20 5.21 5.22 5.23 5.24 5.25 5.26 5.27 5.28 5.29 5.30 5.31 5.32 5.33 5.34 5.35 5.36 5.37 5.38 5.39 5.40 5.41 5.42 5.43 5.44 5.45 5.46 5.47 Artem Cherkasov, Michael Hsing, Roya Zoraghi, Leonard J Foster, Raymond H See, Nikolay Stoynov, Jihong Jiang, Sukhbir Kaur, Tian Lian, Linda Jackson, Huansheng Gong, Rick Swayze, Emily Amandoron, Farhad Hormozdiari, Phuong Dao, Cenk Sahinalp, Osvaldo Santos-Filho, Peter Axerio-Cilies, Kendall Byler, William R McMaster, Robert C Brunham, B Brett Finlay, Neil E Reiner
Mapping the protein interaction network in methicillin-resistant Staphylococcus aureus.
J Proteome Res: 2011, 10(3);1139-50
[PubMed:21166474] [WorldCat.org] [DOI] (I p) - ↑ Stephan Michalik, Jörg Bernhardt, Andreas Otto, Martin Moche, Dörte Becher, Hanna Meyer, Michael Lalk, Claudia Schurmann, Rabea Schlüter, Holger Kock, Ulf Gerth, Michael Hecker
Life and death of proteins: a case study of glucose-starved Staphylococcus aureus.
Mol Cell Proteomics: 2012, 11(9);558-70
[PubMed:22556279] [WorldCat.org] [DOI] (I p)