Jump to navigation
Jump to search
NCBI: 10-JUN-2013
⊟Summary[edit | edit source]
- organism: Staphylococcus aureus COL
- locus tag: SACOL1102 [new locus tag: SACOL_RS05630 ]
- pan locus tag?: SAUPAN003317000
- symbol: pdhA
- pan gene symbol?: pdhA
- synonym:
- product: pyruvate dehydrogenase complex E1 component subunit alpha
⊟Genome View[edit | edit source]
⊟Gene[edit | edit source]
⊟General[edit | edit source]
- type: CDS
- locus tag: SACOL1102 [new locus tag: SACOL_RS05630 ]
- symbol: pdhA
- product: pyruvate dehydrogenase complex E1 component subunit alpha
- replicon: chromosome
- strand: +
- coordinates: 1112070..1113182
- length: 1113
- essential: unknown other strains
⊟Accession numbers[edit | edit source]
- Gene ID: 3237845 NCBI
- RefSeq: YP_185966 NCBI
- BioCyc: see SACOL_RS05630
- MicrobesOnline: 912570 MicrobesOnline
⊟Phenotype[edit | edit source]
Share your knowledge and add information here. [edit]
⊟DNA sequence[edit | edit source]
- 1
61
121
181
241
301
361
421
481
541
601
661
721
781
841
901
961
1021
1081ATGGCTCCTAAGTTACAAGCCCAATTCGATGCAGTAAAAGTTTTAAATGATACTCAATCG
AAATTTGAAATGGTTCAAATTTTGGATGAGAATGGTAACGTCGTAAATGAAGACTTAGTA
CCTGATCTTACGGATGAACAATTAGTGGAATTAATGGAAAGAATGGTATGGACTCGTATC
CTTGATCAACGTTCTATCTCATTAAACAGACAAGGACGTTTAGGTTTCTATGCACCAACT
GCTGGTCAAGAAGCATCACAATTAGCGTCACAATACGCTTTAGAAAAAGAAGATTACATT
TTACCGGGATACAGAGATGTTCCTCAAATTATTTGGCATGGTTTACCATTAACTGAAGCT
TTCTTATTCTCAAGAGGTCACTTCAAAGGAAATCAATTCCCTGAAGGCGTTAATGCATTA
AGCCCACAAATTATTATCGGTGCACAATACATTCAAGCTGCTGGTGTTGCATTTGCACTT
AAAAAACGTGGTAAAAATGCAGTTGCAATCACTTACACTGGTGACGGTGGTTCTTCACAA
GGTGATTTCTACGAAGGTATTAACTTTGCAGCAGCTTATAAAGCACCTGCAATTTTCGTT
ATTCAAAACAATAACTATGCAATTTCAACACCAAGAAGCAAGCAAACTGCTGCTGAAACA
TTAGCTCAAAAAGCAATTGCTGTAGGTATTCCTGGTATCCAAGTTGATGGTATGGATGCG
TTAGCTGTATATCAAGCAACTAAAGAAGCACGTGACCGCGCAGTTGCAGGTGAAGGTCCA
ACATTAATTGAAACTATGACATATCGTTATGGTCCTCATACAATGGCTGGTGACGATCCA
ACTCGTTACAGAACTTCAGACGAAGATGCTGAATGGGAGAAAAAAGACCCATTAGTACGT
TTCCGTAAATTCCTTGAAAACAAAGGTTTATGGAATGAAGACAAAGAAAATGAAGTTATT
GAACGTGCAAAAGCTGATATTAAAGCAGCAATTAAAGAGGCTGATAACACTGAAAAACAA
ACTGTTACTTCTCTAATGGAAATTATGTATGAAGATATGCCTCAAAACTTAGCAGAACAA
TATGAAATTTACAAAGAGAAGGAGTCGAAGTAA60
120
180
240
300
360
420
480
540
600
660
720
780
840
900
960
1020
1080
1113
⊟Protein[edit | edit source]
⊟General[edit | edit source]
- locus tag: SACOL1102 [new locus tag: SACOL_RS05630 ]
- symbol: PdhA
- description: pyruvate dehydrogenase complex E1 component subunit alpha
- length: 370
- theoretical pI: 4.62739
- theoretical MW: 41382.3
- GRAVY: -0.484054
⊟Function[edit | edit source]
- reaction: EC 1.2.4.1? ExPASyPyruvate dehydrogenase (acetyl-transferring) Pyruvate + [dihydrolipoyllysine-residue acetyltransferase] lipoyllysine = [dihydrolipoyllysine-residue acetyltransferase] S-acetyldihydrolipoyllysine + CO2
- TIGRFAM: Energy metabolism Pyruvate dehydrogenase pyruvate dehydrogenase (acetyl-transferring) E1 component, alpha subunit (TIGR03181; EC 1.2.4.1; HMM-score: 488)and 5 moreEnergy metabolism Pyruvate dehydrogenase pyruvate dehydrogenase (acetyl-transferring) E1 component, alpha subunit (TIGR03182; EC 1.2.4.1; HMM-score: 217.3)Biosynthesis of cofactors, prosthetic groups, and carriers Other 1-deoxy-D-xylulose-5-phosphate synthase (TIGR00204; EC 2.2.1.7; HMM-score: 21.6)Biosynthesis of cofactors, prosthetic groups, and carriers Thiamine 1-deoxy-D-xylulose-5-phosphate synthase (TIGR00204; EC 2.2.1.7; HMM-score: 21.6)Biosynthesis of cofactors, prosthetic groups, and carriers Pyridoxine 1-deoxy-D-xylulose-5-phosphate synthase (TIGR00204; EC 2.2.1.7; HMM-score: 21.6)Energy metabolism TCA cycle oxoglutarate dehydrogenase (succinyl-transferring), E1 component (TIGR00239; EC 1.2.4.2; HMM-score: 16.7)
- TheSEED :
- Pyruvate dehydrogenase E1 component alpha subunit (EC 1.2.4.1)
Carbohydrates Central carbohydrate metabolism Dehydrogenase complexes Pyruvate dehydrogenase E1 component alpha subunit (EC 1.2.4.1)and 1 more - PFAM: THDP-binding (CL0254) E1_dh; Dehydrogenase E1 component (PF00676; HMM-score: 352.9)and 3 moreDXP_synthase_N; 1-deoxy-D-xylulose-5-phosphate synthase (PF13292; HMM-score: 21.3)TPP_enzyme_C; Thiamine pyrophosphate enzyme, C-terminal TPP binding domain (PF02775; HMM-score: 16.8)no clan defined RNase_J_C; Ribonuclease J C-terminal domain (PF17770; HMM-score: 13)
⊟Structure, modifications & cofactors[edit | edit source]
- domains:
- modifications:
- cofactors: thiamine diphosphate
- effectors:
⊟Localization[edit | edit source]
- PSORTb: Cytoplasmic
- Cytoplasmic Score: 9.97
- Cytoplasmic Membrane Score: 0
- Cellwall Score: 0.01
- Extracellular Score: 0.02
- Internal Helices: 0
- DeepLocPro: Cytoplasmic
- Cytoplasmic Score: 0.8799
- Cytoplasmic Membrane Score: 0.0412
- Cell wall & surface Score: 0.0014
- Extracellular Score: 0.0775
- LocateP: Intracellular
- Prediction by SwissProt Classification: Cytoplasmic
- Pathway Prediction: No pathway
- Intracellular possibility: 1
- Signal peptide possibility: -1
- N-terminally Anchored Score: -1
- Predicted Cleavage Site: No CleavageSite
- SignalP: no predicted signal peptide
- SP(Sec/SPI): 0.000996
- TAT(Tat/SPI): 0.00008
- LIPO(Sec/SPII): 0.000142
- predicted transmembrane helices (TMHMM): 0
⊟Accession numbers[edit | edit source]
⊟Protein sequence[edit | edit source]
- MAPKLQAQFDAVKVLNDTQSKFEMVQILDENGNVVNEDLVPDLTDEQLVELMERMVWTRILDQRSISLNRQGRLGFYAPTAGQEASQLASQYALEKEDYILPGYRDVPQIIWHGLPLTEAFLFSRGHFKGNQFPEGVNALSPQIIIGAQYIQAAGVAFALKKRGKNAVAITYTGDGGSSQGDFYEGINFAAAYKAPAIFVIQNNNYAISTPRSKQTAAETLAQKAIAVGIPGIQVDGMDALAVYQATKEARDRAVAGEGPTLIETMTYRYGPHTMAGDDPTRYRTSDEDAEWEKKDPLVRFRKFLENKGLWNEDKENEVIERAKADIKAAIKEADNTEKQTVTSLMEIMYEDMPQNLAEQYEIYKEKESK
⊟Experimental data[edit | edit source]
- experimentally validated: PeptideAtlas
- protein localization: Cytoplasmic [1] [2] [3] [4] [5]
- quantitative data / protein copy number per cell: 6109 [6]
- interaction partners:
SACOL2657 (arcA) arginine deiminase [7] (data from MRSA252) SACOL0842 (eno) phosphopyruvate hydratase [7] (data from MRSA252) SACOL1199 (ftsZ) cell division protein FtsZ [7] (data from MRSA252) SACOL0593 (fusA) elongation factor G [7] (data from MRSA252) SACOL1513 (hup) DNA-binding protein HU [7] (data from MRSA252) SACOL1741 (icd) isocitrate dehydrogenase [7] (data from MRSA252) SACOL1103 (pdhB) pyruvate dehydrogenase complex E1 component subunit beta [7] (data from MRSA252) SACOL1104 (pdhC) branched-chain alpha-keto acid dehydrogenase E2 [7] (data from MRSA252) SACOL1105 (pdhD) dihydrolipoamide dehydrogenase [7] (data from MRSA252) SACOL1745 (pyk) pyruvate kinase [7] (data from MRSA252) SACOL0584 (rplA) 50S ribosomal protein L1 [7] (data from MRSA252) SACOL2236 (rplB) 50S ribosomal protein L2 [7] (data from MRSA252) SACOL2239 (rplC) 50S ribosomal protein L3 [7] (data from MRSA252) SACOL2227 (rplE) 50S ribosomal protein L5 [7] (data from MRSA252) SACOL2224 (rplF) 50S ribosomal protein L6 [7] (data from MRSA252) SACOL0585 (rplJ) 50S ribosomal protein L10 [7] (data from MRSA252) SACOL2220 (rplO) 50S ribosomal protein L15 [7] (data from MRSA252) SACOL1257 (rplS) 50S ribosomal protein L19 [7] (data from MRSA252) SACOL1702 (rplU) 50S ribosomal protein L21 [7] (data from MRSA252) SACOL2234 (rplV) 50S ribosomal protein L22 [7] (data from MRSA252) SACOL2237 (rplW) 50S ribosomal protein L23 [7] (data from MRSA252) SACOL1274 (rpsB) 30S ribosomal protein S2 [7] (data from MRSA252) SACOL2233 (rpsC) 30S ribosomal protein S3 [7] (data from MRSA252) SACOL1769 (rpsD) 30S ribosomal protein S4 [7] (data from MRSA252) SACOL2206 (rpsI) 30S ribosomal protein S9 [7] (data from MRSA252) SACOL2230 (rpsQ) 30S ribosomal protein S17 [7] (data from MRSA252) SACOL1448 (sucB) dihydrolipoamide succinyltransferase [7] (data from MRSA252) SACOL1276 (tsf) elongation factor Ts [7] (data from MRSA252) SACOL0594 (tuf) elongation factor Tu [7] (data from MRSA252) SACOL0731 LysR family transcriptional regulator [7] (data from MRSA252) SACOL0973 fumarylacetoacetate hydrolase [7] (data from MRSA252) SACOL1759 universal stress protein [7] (data from MRSA252) SACOL2173 alkaline shock protein 23 [7] (data from MRSA252)
⊟Expression & Regulation[edit | edit source]
⊟Operon[edit | edit source]
- MicrobesOnline: pdhA > pdhB > pdhC > pdhD
⊟Regulation[edit | edit source]
- regulator:
⊟Transcription pattern[edit | edit source]
- S.aureus Expression Data Browser: data available for NCTC8325
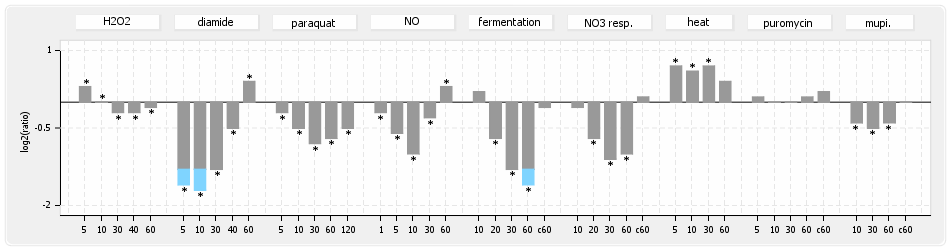
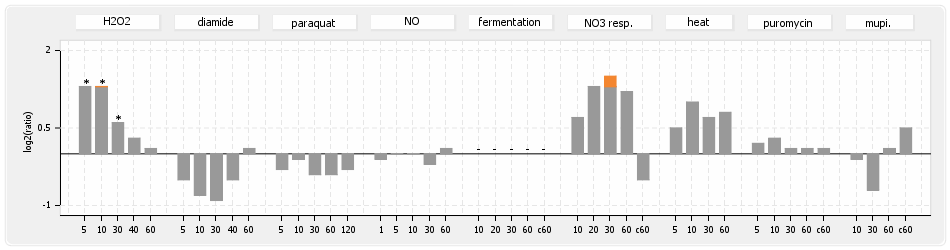
⊟Protein synthesis (provided by Aureolib)[edit | edit source]
⊟Protein stability[edit | edit source]
- half-life: 20.57 h [8]
⊟Biological Material[edit | edit source]
⊟Mutants[edit | edit source]
⊟Expression vector[edit | edit source]
⊟lacZ fusion[edit | edit source]
⊟GFP fusion[edit | edit source]
⊟two-hybrid system[edit | edit source]
⊟FLAG-tag construct[edit | edit source]
⊟Antibody[edit | edit source]
⊟Other Information[edit | edit source]
You can add further information about the gene and protein here. [edit]
⊟Literature[edit | edit source]
⊟References[edit | edit source]
- ↑ Dörte Becher, Kristina Hempel, Susanne Sievers, Daniela Zühlke, Jan Pané-Farré, Andreas Otto, Stephan Fuchs, Dirk Albrecht, Jörg Bernhardt, Susanne Engelmann, Uwe Völker, Jan Maarten van Dijl, Michael Hecker
A proteomic view of an important human pathogen--towards the quantification of the entire Staphylococcus aureus proteome.
PLoS One: 2009, 4(12);e8176
[PubMed:19997597] [WorldCat.org] [DOI] (I e) - ↑ Kristina Hempel, Jan Pané-Farré, Andreas Otto, Susanne Sievers, Michael Hecker, Dörte Becher
Quantitative cell surface proteome profiling for SigB-dependent protein expression in the human pathogen Staphylococcus aureus via biotinylation approach.
J Proteome Res: 2010, 9(3);1579-90
[PubMed:20108986] [WorldCat.org] [DOI] (I p) - ↑ Annette Dreisbach, Kristina Hempel, Girbe Buist, Michael Hecker, Dörte Becher, Jan Maarten van Dijl
Profiling the surfacome of Staphylococcus aureus.
Proteomics: 2010, 10(17);3082-96
[PubMed:20662103] [WorldCat.org] [DOI] (I p) - ↑ Kristina Hempel, Florian-Alexander Herbst, Martin Moche, Michael Hecker, Dörte Becher
Quantitative proteomic view on secreted, cell surface-associated, and cytoplasmic proteins of the methicillin-resistant human pathogen Staphylococcus aureus under iron-limited conditions.
J Proteome Res: 2011, 10(4);1657-66
[PubMed:21323324] [WorldCat.org] [DOI] (I p) - ↑ Andreas Otto, Jan Maarten van Dijl, Michael Hecker, Dörte Becher
The Staphylococcus aureus proteome.
Int J Med Microbiol: 2014, 304(2);110-20
[PubMed:24439828] [WorldCat.org] [DOI] (I p) - ↑ Daniela Zühlke, Kirsten Dörries, Jörg Bernhardt, Sandra Maaß, Jan Muntel, Volkmar Liebscher, Jan Pané-Farré, Katharina Riedel, Michael Lalk, Uwe Völker, Susanne Engelmann, Dörte Becher, Stephan Fuchs, Michael Hecker
Costs of life - Dynamics of the protein inventory of Staphylococcus aureus during anaerobiosis.
Sci Rep: 2016, 6;28172
[PubMed:27344979] [WorldCat.org] [DOI] (I e) - ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 7.15 7.16 7.17 7.18 7.19 7.20 7.21 7.22 7.23 7.24 7.25 7.26 7.27 7.28 7.29 7.30 7.31 7.32 Artem Cherkasov, Michael Hsing, Roya Zoraghi, Leonard J Foster, Raymond H See, Nikolay Stoynov, Jihong Jiang, Sukhbir Kaur, Tian Lian, Linda Jackson, Huansheng Gong, Rick Swayze, Emily Amandoron, Farhad Hormozdiari, Phuong Dao, Cenk Sahinalp, Osvaldo Santos-Filho, Peter Axerio-Cilies, Kendall Byler, William R McMaster, Robert C Brunham, B Brett Finlay, Neil E Reiner
Mapping the protein interaction network in methicillin-resistant Staphylococcus aureus.
J Proteome Res: 2011, 10(3);1139-50
[PubMed:21166474] [WorldCat.org] [DOI] (I p) - ↑ Stephan Michalik, Jörg Bernhardt, Andreas Otto, Martin Moche, Dörte Becher, Hanna Meyer, Michael Lalk, Claudia Schurmann, Rabea Schlüter, Holger Kock, Ulf Gerth, Michael Hecker
Life and death of proteins: a case study of glucose-starved Staphylococcus aureus.
Mol Cell Proteomics: 2012, 11(9);558-70
[PubMed:22556279] [WorldCat.org] [DOI] (I p)